
Is nitrogen dioxide a nonpolar or polar molecule?Īs a gas, NO2 (or nitrogen dioxide) can easily convert to NO2 (nitrite ion), demonstrating its polar nature.


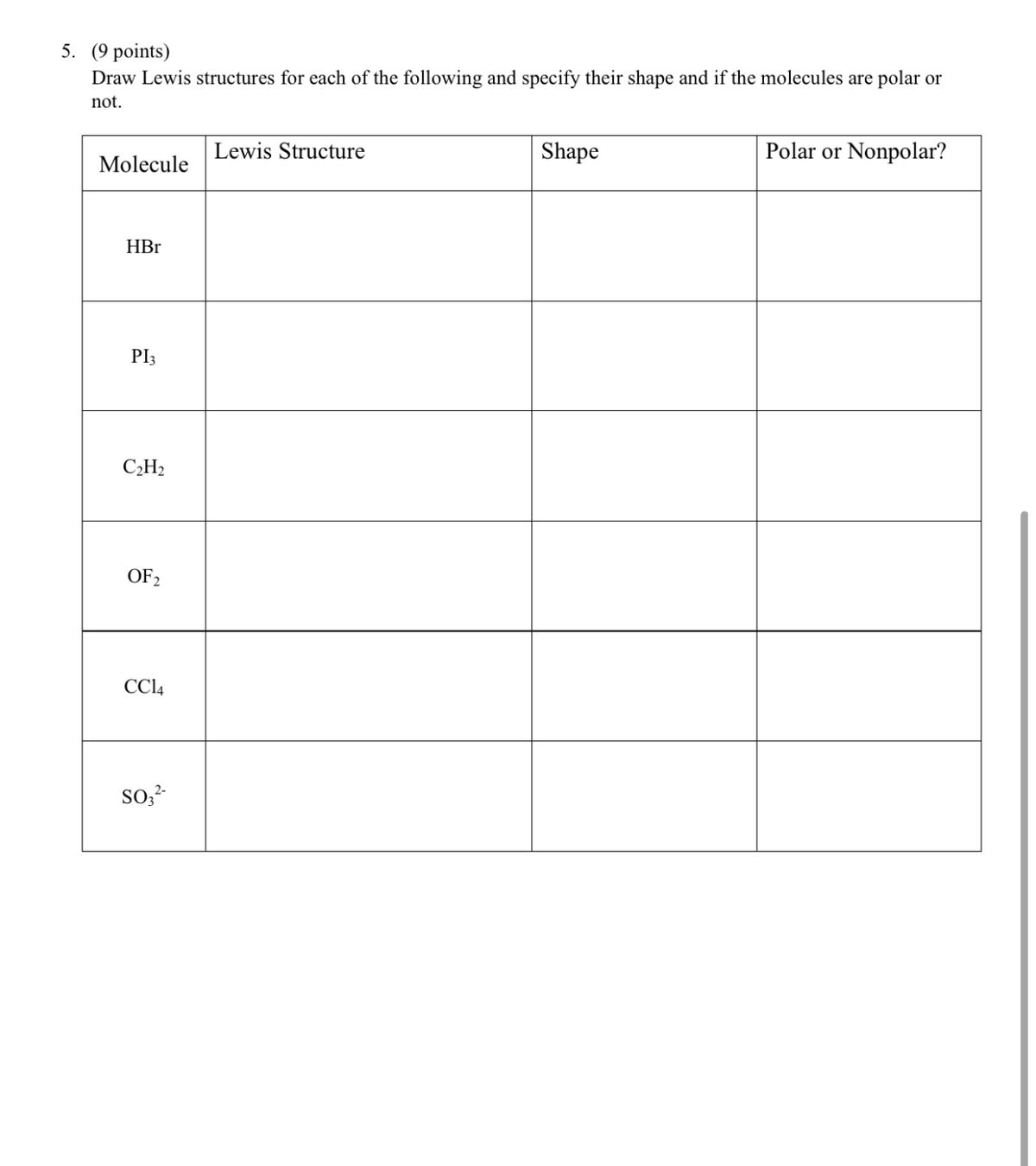
There is a net dipole in CH3OH because the sharing is not equal. To determine whether CH3OH is polar, however, we must consider molecular geometry. It does not appear to be a symmetrical molecule when you look at the Lewis structure for CH3OH. They are forced to the opposite side of the Carbon atom when they do, giving CO2 a linear molecular shape.īecause it has a linear molecular geometry, the CO2 bond angle will be 180 degrees. This is because the Electron Pairs of Valence Shell will Repel each other. As a result, they will be pushed down, giving the CH4 molecule a tetrahedral molecular geometry or shape. These electrons will repel the electron clouds of the two Oxygen atoms at the end, according to VSEPR Theory (Valence Shell Electron Pair Repulsion Theory). The molecular geometry of NH3 is trigonal pyramidal.īecause the hydrogen atoms are repelled by the Nitrogen atom’s lone pair of electrons, the NH3 bond angles are 107 degrees. Tetrahedral What is the shape of an nh3 molecule?Ī quick overview of NH3’s molecular geometry, as well as a description of its NH3 bond angles. When they form a bond by each contributing one electron and then equally sharing the bond pair electrons.Īs a result, there is no charge separation in the molecule, and as a result, the hydrogen molecule has a non-polar property. What is the shape and polarity of H2O? trigonal planer, nonpolar bent, polar Is there a polar molecule in h2?īecause a hydrogen molecule is made up of two electronegativity-equal hydrogen atoms. What is BI3’s shape and polarity? It’s linear, nonpolar, and nonpolar. What is the shape and polarity of H2? Molecular Geometry and PolarityAB What is the shape and polarity of BeBr2? It’s linear, nonpolar, and nonpolar. Why Fstream H is used in C++? What is the shape of h2?.Because it has a Bent molecular geometry, the H2S bond angle will be around 109.5 degrees. What is h2s’ shape and bond angle?Ī quick overview of H2S’ molecular geometry, as well as a description of H2S bond angles (note that H2S has a precise bond angle of 92.1 degrees (see ). In c2h2, how many sigma and pi bonds do you have? As a result, Acetylene (a common name) has three sigma and two pi bonds in its linear structure for Ethyne.Ĭ-H is a three-sigma bond calculation. Because its two carbon atoms are bonded together in a triple bond, acetylene is unsaturated as an alkyne.Īll four atoms are drawn in the same straight line by the carbon–carbon triple bond, with 180° CCH bond angles. What is Ethyne’s structure? C2H2What is the shape of acetylene molecule Commercial grade acetylene has a distinct odor due to impurities, but pure acetylene is odorless. Because it has a linear geometry, the C2H2 bond angle will be 180 degrees. As a result, they will be pushed apart, giving the molecule a linear molecular geometry or shape. The electron clouds on the atoms around C will repel each other, according to VSEPR Theory (Valence Shell Electron Pair Repulsion Theory).


 0 kommentar(er)
0 kommentar(er)
